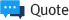AbClon

Immunogenicity Service
Immunogenicity is one of the key issues in the development of recombinant protein drugs. Recom-
binant protein medications can cause unwanted immune responses to the patient being treated,
and this reaction is not only threatening the patient's life, but can also have a variety of effects on
the treatment. These immunological reactions are complex, and in addition to producing antibodies,
potential side effects from reactions such as T cell activation or innate immune response activation
can occur. For this reason, the Korea Food & Drug Administration (KFDA) also provides
guidelines and regulations on immunogenicity. The immunogenicity induction is indicated by the protein sequence and structure. If immunogenicity occurs at the clinical stage without confirmation at the early stage of the development of the recombinant protein drug, problems may arise that must be developed from the initial stage of development of the recombinant protein drug. Identifying immunogenicity early in the development of protein drugs is an important factor that not only increases the effectiveness of drug development, but also determines the success of drug development.
binant protein medications can cause unwanted immune responses to the patient being treated,
and this reaction is not only threatening the patient's life, but can also have a variety of effects on
the treatment. These immunological reactions are complex, and in addition to producing antibodies,
potential side effects from reactions such as T cell activation or innate immune response activation
can occur. For this reason, the Korea Food & Drug Administration (KFDA) also provides
guidelines and regulations on immunogenicity. The immunogenicity induction is indicated by the protein sequence and structure. If immunogenicity occurs at the clinical stage without confirmation at the early stage of the development of the recombinant protein drug, problems may arise that must be developed from the initial stage of development of the recombinant protein drug. Identifying immunogenicity early in the development of protein drugs is an important factor that not only increases the effectiveness of drug development, but also determines the success of drug development.

Immunogenicity factor
- Factors related to medicines
- - protein sequence and structure
- - Glycosylation, deamination, oxidation, etc.
- - Impurities, aggregation and degradation
- - Formulation and storage conditions
- Treatment-related factors
- - Treatment period
- - Treatment concentration
- - Treatment route and frequency
Type of service
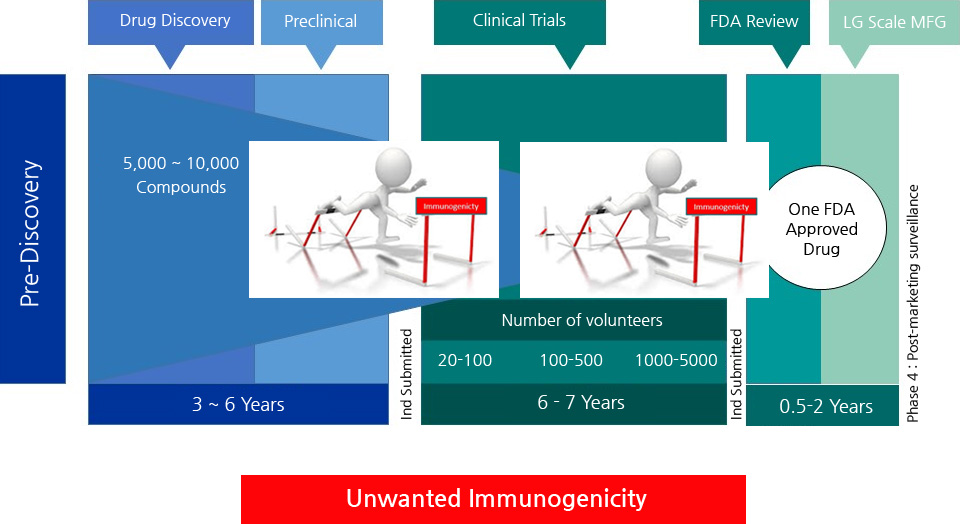
- In silico
- - T cell epitope immunogenicity prediction
- - immunogenicity elimination
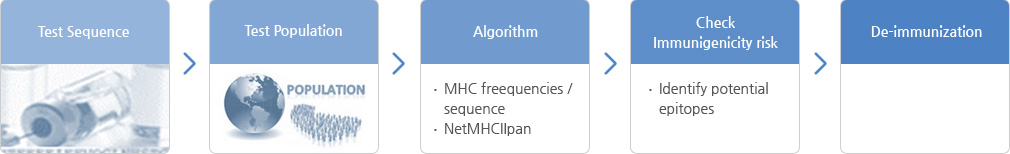
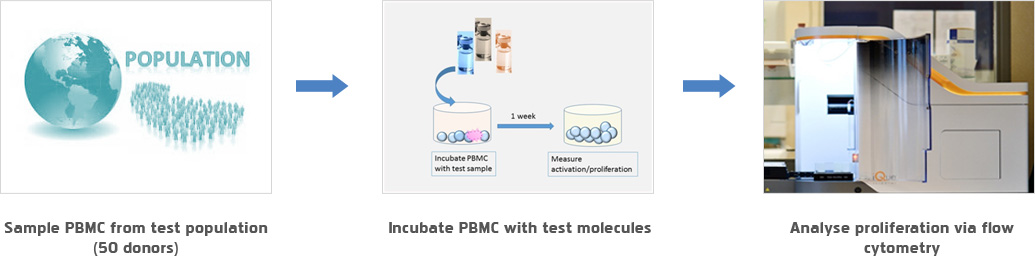
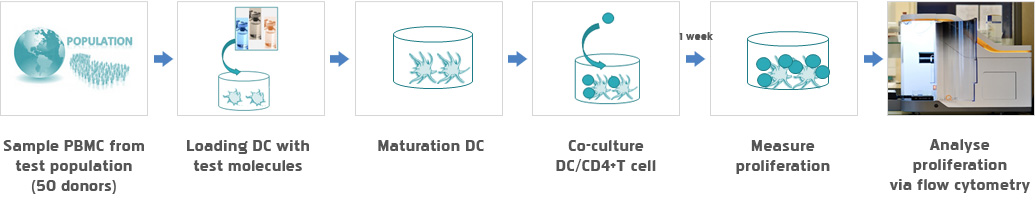
- In vitro
- - PBMC analysis
- - DC T cell analysis
Service Flow
In silico
In vitro
- PBMC
- DC T cell
+82-2-2109-1294
kdlee@abclon.com